Table of Contents
Introduction
Heat is transmitted in several ways. Please read the whole article to know more about it. Here are the highlights of heat transmission:
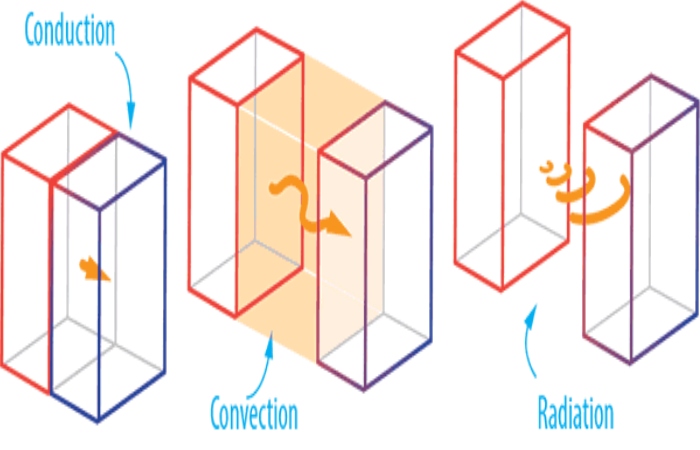
Conduction
The concept of heat transmission by conduction reflects the natural movement of energy from one particle to another by communication between particles. And reflects the transmission and distribution of thermal energy from one atom to another within a single material.
The delivery method is highly effective in solids. Still, it can also occur with liquids. And a practical example of conduction is heating a spoon when placed in a bowl containing hot soup. As the heat will move from soup to spoon.
Convection
The concept of heat transmission by thermal load reflects the movement of internal energy to and from the body through the physical movement resulting from the fluid surrounding the body, which transmits inner power through its mass.
Although the heat transfer process begins with the conduction between the body and the liquid, a more significant amount of energy transmission occurs due to fluid movement.
Convection can occur automatically, naturally, or freely by forming pregnancy cells or created by pushing fluid through the body or by pressing the body through fluids.
Radiation
Radiation heat transmission does not require any link between the heat source and the body, to which heat is transmitted, unlike the methods of load and conduction that require the presence of a heat transfer material.
In this way, the heat can transfer through the vacuum through thermal radiation. For example, the transfer of heat from the sun to the human without having to touch it directly.
The process of radiation transmission does not require an exchange of masses or a medium between objects.
Applications on Heat transmission Methods2024-03-28
Here are the highlights of heat transmission methods:
The Principle of Thermal Exchange
This principle represents the process of transferring heat from a higher temperature body to an object with a lower temperature so that temperatures are equal even to balance. For example, when a cup of coffee is placed at 80°c in the middle at a temperature of 26°c. The cup temperature will drop to an even level with the surrounding medium.
Newton Cooling Act
This law indicates that objects at different temperatures will reach a typical temperature with the temperature of the surrounding medium. As the change in the temperature of a hot object becomes lower as the surrounding medium heats.
In contrast, the difference in cold body temperature increases when the temperature of the surrounding medium decreases. For example, the change in the temperature of a hot apple pie placed in the fridge is more significant than when placed at room temperature.
Greenhouse Phenomenon
This phenomenon occurs in greenhouses. Through which plants can absorb solar radiation to remain warm, especially in winter. As transparent glass allows the passage of visible light in the form of short waves but prevents the release of long-wave radiation that remains trapped inside.
Conclusion
The heat between objects transmits several ways, most notably conduction that reflects the transfer of thermal energy in one particle from one particle to another. Radiation that does not need a substance to transmit heat, and pregnancy that reflects heat transfer in the body a result of physical movement. As a result, the cup temperature will drop even with the surrounding medium.
Applications include heat transmission, thermal exchange, newton’s cooling law, and the phenomenon of glass or greenhouse. After reading the whole article, you will get a brief idea of heat transmission.